Hvert år får 1.400 danskere stillet diagnosen rectumcancer (RC), defineret som adenokarcinom i de nederste 15 cm af tarmen, mens dobbelt så mange får konstateret coloncancer (CC).
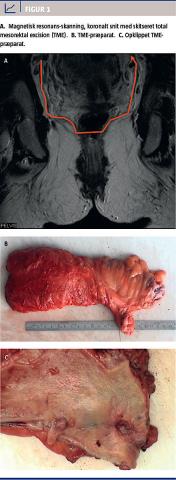
Tidligere var chancen for helbredelse mindre for patienter med RC end for patienter med CC, men optimering af den kirurgiske teknik med indførelse af total mesorektal excision (TME) (Figur 1) og mere korrekt stadieinddeling med magnetisk resonans-skanning, indførelse af tværfaglig konference og præoperativ kort strålebehandling (RT) eller kemostrålebehandling (KRT) til højrisikopatienter har udlignet denne forskel [1] og har reduceret andelen af lokalrecidiver fra 40% til ca. 10% [2]. Imidlertid dør omkring halvdelen af patienterne fortsat pga. fjernmetastasering, hvorfor fokus nu er rettet mod at hindre systemisk spredning.
Hos patienter med CC i stadie 3 og højrisikostadie 2 (primært gennemvækst af tarmen) øger postoperativ behandling med 5-fluorouracil (5FU) eller et af de perorale analoger (capecitabin, tegafur-uracil eller S1) overlevelsen med ti procentpoint, og ved tillæg af oxaliplatin med yderligere fem procentpoint [3].
I mange lande har man tolket, at der ikke er biologisk eller klinisk forskel på adenokarcinom i colon og rectum, og man har derfor indført adjuverende kemoterapi til patienter med RC både i stadie 2 og stadie 3, uden at der har været tilstrækkelig videnskabelig evidens for en sådan beslutning [4].
Derfor er det af stor betydning, at Cochranesamarbejdet [5] for nylig har publiceret en metaanalyse, hvor man vurderer adjuverende kemoterapi hos patienter med RC.
Formålet med denne artikel er at præsentere hovedresultaterne af denne oversigt og perspektivere resultaterne til danske forhold.
COCHRANEMETAANALYSEN
Forfatterne foretog en systematisk litteratursøgning i en række elektroniske databaser frem til marts 2011. De inkluderede alle studier, hvor patienterne med radikalt reseceret ikkemetastaserende RC blev randomiseret til ingen postoperativ kemoterapi eller postoperativ adjuverende kemoterapi. Der blev ikke taget hensyn til, om patienterne havde fået præoperativ, postoperativ eller ingen supplerende strålebehandling. Forfatterne fandt 21 studier med ca. 16.000 patienter med kolorektalcancer, hvoraf knap 10.000 patienter havde adenokarcinom i rectum.
Det primære effektmål var hazard ratio (HR) mellem risiko for event (recidiv eller død) hos behandlede (adjuverende kemoterapi) og ikkebehandlede patienter.

RESULTATER
I alle de inkluderede studier fik patienterne adjuverende 5FU-baseret kemoterapi. I ingen af studierne inkluderede man patienter, der fik behandling med de nyere cytostatika (oxaliplatin, irinotecan) eller biologiske stoffer.
Det overordnede resultat af metaanalysen var, at postoperativ 5FU-baseret kemoterapi hos patienter med RC reducerede risikoen for recidiv med 25% (HR 0,75) og risikoen for død med 17% (HR 0,83). Sammenlignelige effekter blev også fundet i subgruppeanalyser, opdelt efter om studiet var et »vestligt« eller et »asiatisk« studie. Det var ikke muligt at vurdere effekten separat for de enkelte TNM-stadier, da disse data kun blev rapporteret i ganske få studier.
Forfatterne konkluderede, at metaanalysen understøtter brugen af 5FU-baseret adjuverende kemoterapi efter radikal fjernelse af RC.
Postoperative adjuvant chemotherapy in rectal cancer operated for cure
Petersen SH1, Harling H2, Kirkeby LT3, Wille-Jørgensen P4, Mocellin S5
1) Colorectal Cancer Group, Bispebjerg Hospital, building 11B, Copenhagen NV, Denmark. 2) Surgical Department K, Bispebjerg Hospital, Copenhagen NV, Denmark. 3) Surg. Department A, Roskilde Hospital, Roskilde, Denmark. 4) Department of Surgical Gastroenterology K, Bispebjerg Hospital, Copenhagen NV, Denmark.
5) Meta-Analysis Unit, Department of Oncological & Surgical Sciences, University of Padova, Padova, Italy
Contact address: Sune Høirup Petersen, Colorectal Cancer Group, Bispebjerg Hospital, building 11B, 23 Bispebjerg Bakke, 2400 Copenhagen NV, Denmark.
SPET0035@bbh.regionh.dk.
Editorial group: Cochrane Colorectal Cancer Group.
Publication status and date: New, comment added to review, published in Issue 3, 2012.
Review content assessed as up-to-date: 31 January 2012.
Citation: Petersen SH, Harling H, Kirkeby LT, Wille-Jørgensen P, Mocellin S. Postoperative adjuvant chemotherapy in
rectal cancer operated for cure. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No.: CD004078. DOI:
10.1002/14651858.CD004078.pub2.
Copyright © 2012 The Cochrane Collaboration. Published by JohnWiley & Sons, Ltd.
Background
Colorectal cancer is one of the most common types of cancer in the Western world. Apart from surgery – which remains the mainstay of treatment for resectable primary tumours – postoperative (i.e., adjuvant) chemotherapy with 5-fluorouracil (5-FU) based regimens is now the standard treatment in Dukes‘ C (TNM stage III) colon tumours i.e. tumours with metastases in the regional lymph nodes but no distant metastases. In contrast, the evidence for recommendations of adjuvant therapy in rectal cancer is sparse. In Europe it is generally acknowledged that locally advanced rectal tumours receive preoperative (i.e., neoadjuvant) downstaging by radiotherapy (or chemoradiotion), whereas in the US postoperative chemoradiotion is considered the treatment of choice in all Dukes´ C rectal cancers. Overall, no universal consensus exists on the adjuvant treatment of surgically resectable rectal carcinoma; moreover, no formal systematic review and meta-analysis has been so far performed on this subject.
Objectives
We undertook a systematic review of the scientific literature from 1975 until March 2011 in order to quantitatively summarize the available evidence regarding the impact of postoperative adjuvant chemotherapy on the survival of patients with surgically resectable rectal cancer. The outcomes of interest were overall survival (OS) and disease-free survival (DFS).
Search Methods
CCCG standard search strategy in defined databases with the following supplementary search. 1. Rect* or colorect* – 2. Cancer or carcinom* or adenocarc* or neoplasm* or tumour – 3. Adjuv* – 4. Chemother* - 5. Postoper*
Selection criteria
Randomised controlled trials (RCT) comparing patients undergoing surgery for rectal cancer who received no adjuvant chemotherapy with those receiving any postoperative chemotherapy regimen.
Data collection and analysis
Two authors extracted data and a third author performed an independent search for verification. The main outcome measure was the hazard ratio (HR) between the risk of event between the treatment arm (adjuvant chemotherapy) and the control arm (no adjuvant chemotherapy). The survival data were either entered directly in RevMan or extrapolated from Kaplan-Meier plots and then entered in RevMan. Due to expected clinical heterogeneity a random effects model was used for creating the pooled estimates of treatment efficacy.
Main results
A total of 21 eligible RCTs were identified and used for meta-analysis purposes. Overall, 16,215 patients with colorectal cancer were enrolled, 9,785 being affected with rectal carcinoma. Considering patients with rectal cancer only, 4,854 cases were randomized to receive potentially curative surgery of the primary tumour plus adjuvant chemotherapy and 4,367 to receive surgery plus observation. The mean number of patients enrolled was 466 (range: 54-1,243 cases). 11 RCTs had been performed inWestern countries and 10 in Japan. All trials used fluoropyrimidine-based chemotherapy (no modern drugs - such as oxaliplatin, irinotecan or biological agents - were tested).
Overall survival (OS) data were available in 21 RCTs and the data available for meta-analysis regarded 9,221 patients: of these, 4854 patients were randomized to adjuvant chemotherapy (treatment arm) and 4,367 patients did not receive adjuvant chemotherapy (control arm). The meta-analysis of these RCTs showed a significant reduction in the risk of death (17%) among patients undergoing postoperative chemotherapy as compared to those undergoing observation (HR=0.83, CI: 0.76-0.91). Between-study heterogeneity was moderate (I-squared=30%) but significant (P=0.09) at the 10% alpha level.
Disease-free survival (DFS) data were reported in 20 RCTs, and the data suitable for meta-analysis included 8,530 patients. Of these, 4,515 patients were randomized to postoperative chemotherapy (treatment arm) and 4,015 patients received no postoperative chemotherapy (control arm). The meta-analysis of these RCTs showed a reduction in the risk of disease recurrence (25%) among patients undergoing adjuvant chemotherapy as compared to those undergoing observation (HR=0.75, CI: 0.68-0.83). Between-study heterogeneity was moderate (I-squared =41%) but significant (P=0.03).
While analyzing both OS and DFS data, sensitivity analyses did not find any difference in treatment effect based on trial sample size or geographical region (Western vs Japanese). Available data were insufficient to investigate on the effect of adjuvant chemotherapy separately in different TNMstages in terms of bothOS andDFS.No plausible source of heterogeneity was formally identified, although variability in treatment regimens and TNM stages of enrolled patients might have played a significant role in the difference of reported results.
Authors’ conclusions
The results of thismeta-analysis support the use of 5-FU based postoperative adjuvant chemotherapy for patients undergoing apparently radical surgery for non-metastatic rectal carcinoma. Available data do not allow us to define whether the efficacy of this treatment is highest in one specific TNM stage. The implementation of modern anti-cancer agents in the adjuvant setting is warranted to improve the results shown by this meta-analysis. Randomized trials of adjuvant chemotherapy for patients receiving preoperative neoadjuvant therapy are also needed in order to define the role of postoperative chemotherapy in the multimodal treatment of resectable rectal cancer.
Postoperative adjuvant chemotherapy in rectal cancer operated for cure
Petersen SH1, Harling H2, Kirkeby LT3, Wille-Jørgensen P4, Mocellin S5
1) Colorectal Cancer Group, Bispebjerg Hospital, building 11B, Copenhagen NV, Denmark. 2) Surgical Department K, Bispebjerg Hospital, Copenhagen NV, Denmark. 3) Surg. Department A, Roskilde Hospital, Roskilde, Denmark. 4) Department of Surgical Gastroenterology K, Bispebjerg Hospital, Copenhagen NV, Denmark.
5) Meta-Analysis Unit, Department of Oncological & Surgical Sciences, University of Padova, Padova, Italy
Contact address: Sune Høirup Petersen, Colorectal Cancer Group, Bispebjerg Hospital, building 11B, 23 Bispebjerg Bakke, 2400 Copenhagen NV, Denmark.
SPET0035@bbh.regionh.dk.
Editorial group: Cochrane Colorectal Cancer Group.
Publication status and date: New, comment added to review, published in Issue 3, 2012.
Review content assessed as up-to-date: 31 January 2012.
Citation: Petersen SH, Harling H, Kirkeby LT, Wille-Jørgensen P, Mocellin S. Postoperative adjuvant chemotherapy in
rectal cancer operated for cure. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No.: CD004078. DOI:
10.1002/14651858.CD004078.pub2.
Copyright © 2012 The Cochrane Collaboration. Published by JohnWiley & Sons, Ltd.
Background
Colorectal cancer is one of the most common types of cancer in the Western world. Apart from surgery – which remains the mainstay of treatment for resectable primary tumours – postoperative (i.e., adjuvant) chemotherapy with 5-fluorouracil (5-FU) based regimens is now the standard treatment in Dukes‘ C (TNM stage III) colon tumours i.e. tumours with metastases in the regional lymph nodes but no distant metastases. In contrast, the evidence for recommendations of adjuvant therapy in rectal cancer is sparse. In Europe it is generally acknowledged that locally advanced rectal tumours receive preoperative (i.e., neoadjuvant) downstaging by radiotherapy (or chemoradiotion), whereas in the US postoperative chemoradiotion is considered the treatment of choice in all Dukes´ C rectal cancers. Overall, no universal consensus exists on the adjuvant treatment of surgically resectable rectal carcinoma; moreover, no formal systematic review and meta-analysis has been so far performed on this subject.
Objectives
We undertook a systematic review of the scientific literature from 1975 until March 2011 in order to quantitatively summarize the available evidence regarding the impact of postoperative adjuvant chemotherapy on the survival of patients with surgically resectable rectal cancer. The outcomes of interest were overall survival (OS) and disease-free survival (DFS).
Search Methods
CCCG standard search strategy in defined databases with the following supplementary search. 1. Rect* or colorect* – 2. Cancer or carcinom* or adenocarc* or neoplasm* or tumour – 3. Adjuv* – 4. Chemother* - 5. Postoper*
Selection criteria
Randomised controlled trials (RCT) comparing patients undergoing surgery for rectal cancer who received no adjuvant chemotherapy with those receiving any postoperative chemotherapy regimen.
Data collection and analysis
Two authors extracted data and a third author performed an independent search for verification. The main outcome measure was the hazard ratio (HR) between the risk of event between the treatment arm (adjuvant chemotherapy) and the control arm (no adjuvant chemotherapy). The survival data were either entered directly in RevMan or extrapolated from Kaplan-Meier plots and then entered in RevMan. Due to expected clinical heterogeneity a random effects model was used for creating the pooled estimates of treatment efficacy.
Main results
A total of 21 eligible RCTs were identified and used for meta-analysis purposes. Overall, 16,215 patients with colorectal cancer were enrolled, 9,785 being affected with rectal carcinoma. Considering patients with rectal cancer only, 4,854 cases were randomized to receive potentially curative surgery of the primary tumour plus adjuvant chemotherapy and 4,367 to receive surgery plus observation. The mean number of patients enrolled was 466 (range: 54-1,243 cases). 11 RCTs had been performed inWestern countries and 10 in Japan. All trials used fluoropyrimidine-based chemotherapy (no modern drugs - such as oxaliplatin, irinotecan or biological agents - were tested).
Overall survival (OS) data were available in 21 RCTs and the data available for meta-analysis regarded 9,221 patients: of these, 4854 patients were randomized to adjuvant chemotherapy (treatment arm) and 4,367 patients did not receive adjuvant chemotherapy (control arm). The meta-analysis of these RCTs showed a significant reduction in the risk of death (17%) among patients undergoing postoperative chemotherapy as compared to those undergoing observation (HR=0.83, CI: 0.76-0.91). Between-study heterogeneity was moderate (I-squared=30%) but significant (P=0.09) at the 10% alpha level.
Disease-free survival (DFS) data were reported in 20 RCTs, and the data suitable for meta-analysis included 8,530 patients. Of these, 4,515 patients were randomized to postoperative chemotherapy (treatment arm) and 4,015 patients received no postoperative chemotherapy (control arm). The meta-analysis of these RCTs showed a reduction in the risk of disease recurrence (25%) among patients undergoing adjuvant chemotherapy as compared to those undergoing observation (HR=0.75, CI: 0.68-0.83). Between-study heterogeneity was moderate (I-squared =41%) but significant (P=0.03).
While analyzing both OS and DFS data, sensitivity analyses did not find any difference in treatment effect based on trial sample size or geographical region (Western vs Japanese). Available data were insufficient to investigate on the effect of adjuvant chemotherapy separately in different TNMstages in terms of bothOS andDFS.No plausible source of heterogeneity was formally identified, although variability in treatment regimens and TNM stages of enrolled patients might have played a significant role in the difference of reported results.
Authors’ conclusions
The results of thismeta-analysis support the use of 5-FU based postoperative adjuvant chemotherapy for patients undergoing apparently radical surgery for non-metastatic rectal carcinoma. Available data do not allow us to define whether the efficacy of this treatment is highest in one specific TNM stage. The implementation of modern anti-cancer agents in the adjuvant setting is warranted to improve the results shown by this meta-analysis. Randomized trials of adjuvant chemotherapy for patients receiving preoperative neoadjuvant therapy are also needed in order to define the role of postoperative chemotherapy in the multimodal treatment of resectable rectal cancer.
DISKUSSION
Siden 1990 har adjuverende behandling været anbefalet til både patienter med CC og patienter med RC. Denne skråsikre anbefaling til patienter med RC er siden blevet kritiseret, idet den videnskabelige evidens var baseret på små studier, hvor patienterne ikke blev opereret med TME-teknik, og hvis der blev givet RT, var det som regel postoperativt. I dag ved man, at præoperativ strålebehandling er mere effektiv og skånsom end postoperativ strålebehandling til patienter med højrisiko-RC [2].
Kun i få studier har man undersøgt effekten af adjuverende kemoterapi efter præoperativ strålebehandling og TME, og kun få studier var dimensionerede til, at man kunne påvise en absolut forskel i femårsoverlevelse på 5%. I en samlet analyse [6] af fem europæiske studier øgede adjuverende 5FU-behandling langtidsoverlevelsen (HR 0,81). Det primære formål med studiet var dog at udvikle et nomogram (baseret på T- og N-stadie, alder, præoperativ RT og adjuverende 5FU-behandling), hvormed man ville kunne forudsige lokalrecidiv og overlevelse og hjælpe med at selektere patienter, som kunne have gavn af adjuverende kemoterapi [7].
Det europæiske studie, der bedst understøtter brugen af adjuverende kemoterapi hos patienter med RC, er QUASAR [8, 9]. I QUASAR inkluderede man mere end 3.000 patienter med kolorektalcancer, hvor inklusionskriteriet var, at indikationen for adjuverende kemoterapi var tvivlsom (primært stadie 2). Patienter med stadie 2 havde lige så stor relativ gevinst af behandling som patienter med stadie 3, og patienter med RC havde lige så stor gevinst som patienter med CC. Adjuverende kemoterapi med 5FU nedsatte den relative risiko for død med 18%. Hos en person med forventet femårsmortalitet på 20% svarer dette til en absolut øgning i overlevelsen på 3,6%.
KONKLUSION
Der er endnu ikke tilstrækkelig evidens til at opnå europæisk konsensus om brugen af 5FU-baseret postoperativ adjuverende kemoterapi efter præoperativ KRT [1], men en samlet vurdering af Cochraneanalysen, de nyeste studier, QUASAR og japanske studier viser alle en relativ reduktion i mortalitet på ca. 20%, svarende til en overlevelsesgevinst på 3-5% for lavrisiko-RC og ca. 10% for højrisiko-RC.
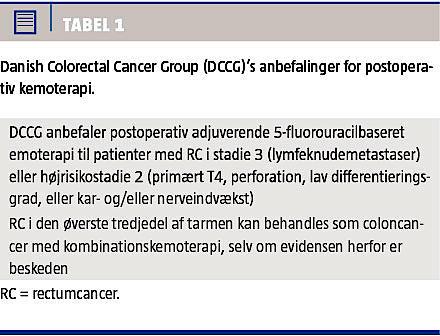
Ovenstående er i overensstemmelse med de opdaterede retningslinjer i Danish Colorectal Cancer Group (Tabel 1), hvori der anbefales adjuverende 5FU-baseret kemoterapi til patienter med RC stadie 3 og højrisikostadie 2 (primært T4, perforation, lav differentieringsgrad eller kar- og/eller nerveindvækst). RC i den øverste tredjedel af tarmen kan behandles som CC med kombinationskemoterapi, selv om evidensen herfor er beskeden [3].
Denne beskedne, men dog klinisk relevante forskel, bør indgå i dialogen mellem patient og onkolog/kirurg. I studier, hvor man undersøger patienters holdninger til at modtage moderat toksisk kemoterapi, anfører mange, at de ønsker behandling, selv hvis overlevelsesgevinsten kun er ca. 1% [10].
Resultaterne af igangværende studier, hvor man undersøger adjuverende 5FU-behandling og kombinationskemoterapi hos patienter, der er optimalt behandlet med præoperativ behandling og radikal TME, afventes.
KORRESPONDANCE: Camilla Qvortrup, Onkologisk Afdeling R, Odense
Universitetshospital, Sdr. Boulevard 29, 5000 Odense C.
E-mail: camilla.qvortrup@ouh.regionsyddanmark.dk
ANTAGET: 12. februar 2013
FØRST PÅ NETTET: 24. juni 2013
INTERESSEKONFLIKTER: ingen. .
TAKSIGELSER: Jan Lindebjerg, Patologisk Afdeling, Vejle Sygehus, takkes for levering af illustrationen.
<h2>LITTERATUR</h2>
<ol type="d">
<li><p></p></li>
<li><p>Bülow S, Harling H, Iversen LH et al. Overlevelsen efter rectumcancer i Danmark er forbedret væsentligt – sekundærpublikation. Ugeskr Læger 2009;171:2735-8.</p></li>
<li><p>Påhlman L, Bohe M, Cedermark B et al. The Swedish rectal cancer registry. <br>Br J Surg 2007;94:1285-92.</p></li>
<li><p>Schmoll HJ, van Cutsem E, Stein A et al. ESMO Consensus Guidelines for <br>management of patients with colon and rectal cancer. Ann Oncol 2012; 23:2479-516.</p></li>
<li><p>Valentini V, Aristei C, Glimelius B et al. Multidisciplinary rectal cancer management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2). <br>Radiother Oncol 2009;92:148-63.</p></li>
<li><p>Petersen SH, Harling H, Kirkeby LT et al. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 2012;3: CD004078. </p></li>
<li><p>Valentini V, van Stiphout RG, Lammering G et al. Nomograms for predicting <br>local recurrence, distant metastases, and overall survival for patients with <br>locally advanced rectal cancer on the basis of European randomized clinical <br>trials. J Clin Oncol 2011;29:3163-72.</p></li>
<li><p>www.predictcancer.org (25. sep 2012).</p></li>
<li><p>QUASAR Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020-9.</p></li>
<li><p>Cunningham D, Starling N. Adjuvant chemotherapy of colorectal cancer. Lancet 2007;370:1980-1.</p></li>
<li><p>Slevin ML, Stubbs L, Plant HJ et al. Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses, and general public. BMJ 1990;300:1458-60.</p></li>
</ol>